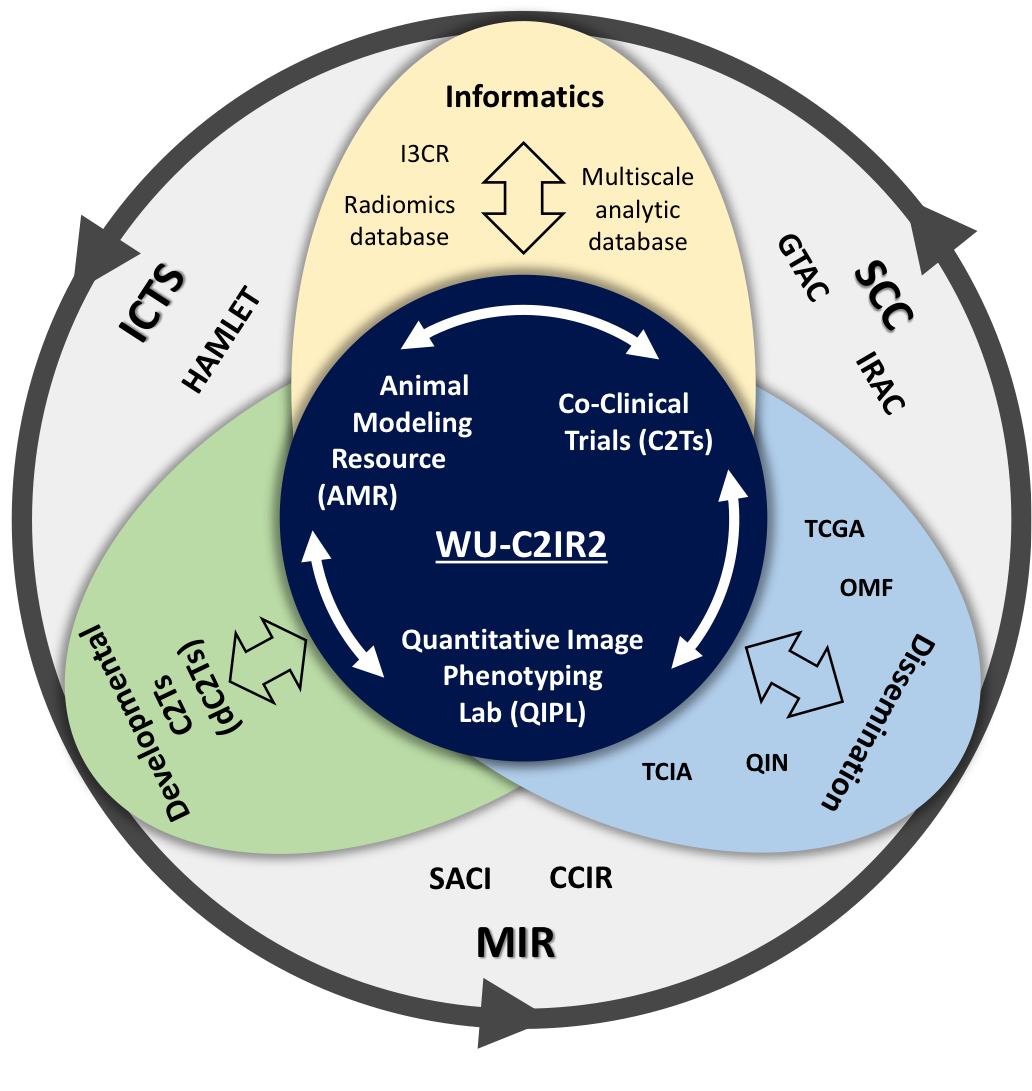
The multidisciplinary research at the WU-C2IR2 is supported by the National Cancer Institute (NCI) co-clinical imaging research program (CIRP) grant U24-CA209837, the Mallinckrodt Institute of Radiology (MIR), and the Siteman Cancer Center (SCC). Details of the CIRP network can be found on the NCI CIRP website.
Research Motivation
Quantitative imaging (QI) is positioned to play a central role in the implementation of precision medicine by providing critical information about tumor’s biology and aid in the design of adaptive therapy trials. To fully realize the potential of QI in precision medicine, there is an unmet need to develop and standerdize QI methodologies that transcend species and modalities. While standardization of QI methods has had a great impact in advancing clinical applications of QI to assess and predict response to therapy, preclinical imaging remains a critical component in the translational pipeline for validating advanced QI methods in drug discovery and assessment of response to therapy. Thus, developing QI standards that transcend species and modalities is critical in advancing the application of QI.
Standardization of preclinical QI has been limited primarily for two reasons: first, use of unrealistic animal models of cancer, such as established cell lines, to validate QI methods, and second, logistical and technical challenges inherent in preclinical imaging. More realistic preclinical cancer models are thought to be transplantable, patient-derived cancer tissue xenografts (PDX). Indeed, the use of PDX can bridge the technical challenges in preclinical imaging by unifying the use of QI across species and modalities to correlate with the biology of the tumor. Importantly, the use of PDX ushers new paradigms involving co-clinical trials where QI applied to PDX can be implemented in the corresponding patient in a clinical setting.
In this context, Washington University Co-Clinical Imaging Research Resource (WU-C2IR2) will develop, optimize, and validate advanced preclinical QI methods using realistic animal models of cancer, such as PDX, to support co-clinical trials. Specifically, the WU-C2IR2 will employ a bi-directional translation of QI methods: (i) clinical-to-preclinical, where established clinical QI protocols can be implemented and then optimized, and validated with preclinical PDX models and (ii) preclinical-to-clinical, where novel, advanced preclinical QI protocols can be optimized and validated with preclinical PDX models prior to translation and implementation in the clinic.