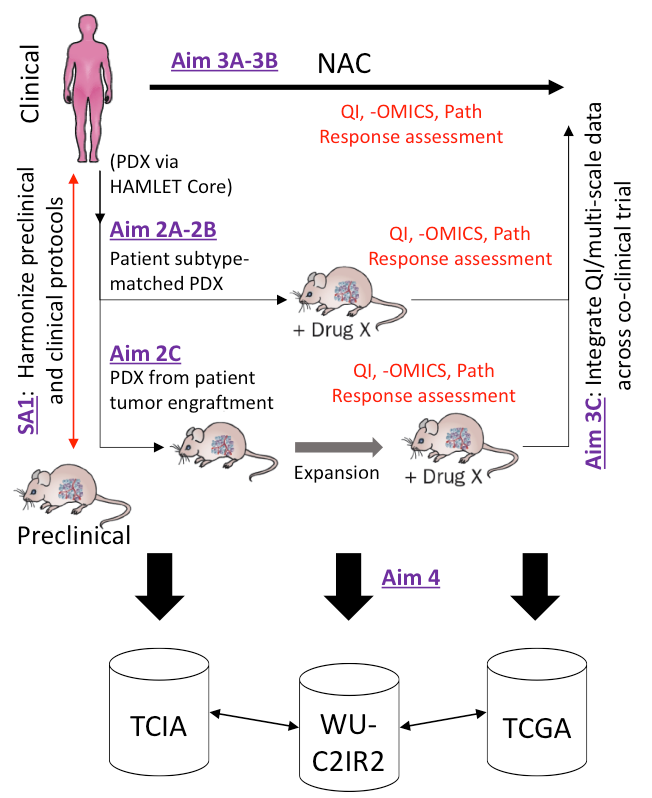
The I2CR2 is working in concert with an active prospective co-clinical trial: Co-Clinical Trial in Triple Negative Breast Cancer Patients with Genoproteomic Discovery.
Triple Negative Breast Cancer (TNBC) is a highly heterogeneous and aggressive tumor characterized by poorer outcome and higher relapse rates compared with other subtypes of breast cancer. While these aggressive tumors are more responsive to chemotherapy, TNBC patients exhibit higher relapse rates than other breast cancer types. It is critical to identify patients who will respond to neoadjuvant chemotherapy (NAC) therapy, and avoid the use of ineffective treatments in nonresponding patients, and develop adaptive treatment strategies.
In collaboration with the HAMLET core of the ICTS, both subtype-matched TNBC PDX and PDX generated from individual patient tumor engraftments will be used to optimize, validate, and then implement co-clinical imaging methods in this co-clinical trial.
The C2IR2 will apply and advance Quantitative Imaging methods in PET/MR imaging protocols to standardize and assess response to therapy in this co-clinical trial. These protocols can be found on this website in the SOP Directory.
Research Plan:
Learn More: https://clinicaltrials.gov/ct2/show/NCT02124902